Solutions provided by Thermal Spray Coating
FST has the experience, knowledge and resources available to provide you with a full support in finding solutions for your specific surface problems or requirements. FST has been successful in providing a wide range of industries with thermal spray processes that have improved their efficiencies, lifespan and reduced their operating costs. FST Thermal Spray Coating systems provide coating solutions for:
Wear resistance
Wear involves physical removal of material from a solid surface by another surface or material.
Adhesion caused by “cold-welding”
When surfaces slide relative to one another, there is a tendency for one material to transfer onto the counterface. This is adhesive wear, that in its most severe form, results in galling and possible seizure of the machine through of cold welding.
SOLUTION: Adhesion is usually combated using dissimilar materials and harder surfaces.
Fretting
Fretting is the result of damage from low amplitude vibration and/or small oscillations between two surfaces in contact with one another. Often, this damage can go unnoticed for a long time.
SOLUTION: FST offers coating solutions that exhibit excellent properties for anti-fretting applications, such as thermal sprayed copper-nickel-indium for the roots of gas turbine blades.
Two body abrasion
A harder body can cut or spall a softer counterface. Abrasion is classified into two primary mechanisms. In two-body abrasion, a hard, rough surface scratches, cuts or spalls a softer surface. In three-body abrasion, a hard third body damages one or both of the sliding surfaces. This is usually due to grit or dirt getting in between the sliding interface.
SOLUTION: Hard and well-adhered coatings and diffusion layers effectively reduce this wear. For effective reduction, the protected surface must be at least 20% harder than the abrasive.
Three body abrasion
A harder body can cut or spall a softer counterface. Abrasion is classified into two primary mechanisms. In two-body abrasion, a hard, rough surface scratches, cuts or spalls a softer surface. In three-body abrasion, a hard third body damages one or both of the sliding surfaces. This is usually due to grit or dirt getting in between the sliding interface.
SOLUTION: Hard and well-adhered coatings and diffusion layers effectively reduce this wear. For effective reduction, the protected surface must be at least 20% harder than the abrasive.
Erosion
Erosion is caused by particulate in gases or liquids striking the surface of a component. The severity of the wear is strongly dependent on the velocity and hardness of the particles, as well as the angle of impact.
SOLUTION: For high-angle attack, a somewhat compliant coating or a very thick coating applied by welding or thermal spray should be selected. For low-angle attack, very hard coatings are preferred.
Impact
Impact is defined as the sudden striking of one object against another with high force. Repetition of impact forces can often cause substrate materials to weaken and crack.
SOLUTION: For impact applications, hard, thick and especially tough materials are required. This is best achieved with systems that are metallurgically bonded to the substrate, such as PTA overlays or fused thermal spray coatings.
Cavitation
Cavitation wear occurs on surfaces exposed to fluids in which entrained bubbles collapse at or near the surface. The collapse releases a jet of the fluid that impacts the surface, causing severe local ‘hammering’.
SOLUTION: Intense cavitation is combated using tough, usually cobalt based materials that strongly work-harden
Fatigue and stress cracking
Fatigue and stress cracking is the direct consequence of changing mechanical stresses.
SOLUTION: Generally, this is best solved by mechanical redesign. However, Thermal spray coatings, especially HVOF, can introduce compressive stresses within the coating that can be used on components exposed to high stress cycling.
 Thermal effects and protection
Thermal effects and protection
Surface technology solutions are used to both insulate components against the effects of high temperatures and promote the conduction of heat uniformly.

Thermal Conduction
Thermal spray coating systems can promote thermal conductivity. Thermal sprayed copper and thermal sprayed aluminum are some examples of a thermally conductive surface. These coatings can not only promote heat transfer, but can evenly distribute the heat for even greater efficiency.
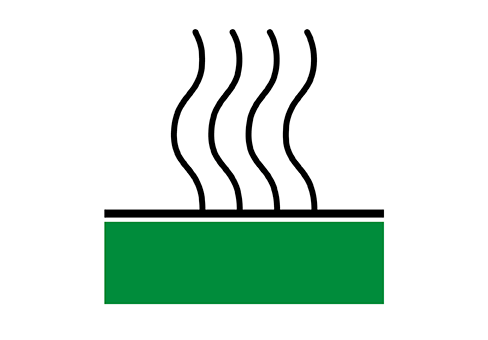
Thermally barrier
Thermally barrier coatings are designed to slow heat transfer and insulate the substrate. The coating systems used today usually consist of two components: a metallic bond coat (usually an MCrAlY) that also acts as an oxidation resistant layer and a top coat of ceramic (usually a yttrium stabilized zirconium oxide). The coating system has a low rate of thermal conductivity and a high thermal expansion coefficient. These coating systems extend the service life of components or allow components to operate at higher temperatures, increasing thermal efficiency.
Corrosion Resistance
Corrosion is an electro-chemical process, which takes place between a metal and the surrounding environment.
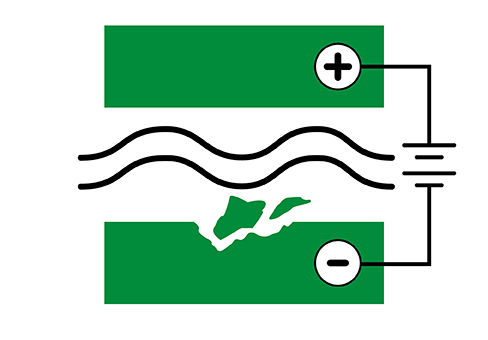
Galvanic corrosion
Galvanic corrosion occurs when dissimilar metals are in the presence of an electrolyte, attacking the more anodic metal. Galvanic corrosion can occur not only when two components of dissimilar metals are in this state, but also on a single component where the individual grains of an alloy have different potentials. Most often, the electrolyte is a saline, acidic or alkaline solution or atmosphere.

Pitting corrosion
Pitting corrosion takes place not in broad areas, but in small cracks, or pits that form as a result of a low availability of oxygen or high concentrations of anions, such as chloride, which can prevent a surface from forming a passive film.

High temperature corrosion
High temperature corrosion is a non-galvanic form of corrosion of a metal exposed to an oxidizing atmosphere, such as sulfur, oxygen or other oxidizing compounds under very high temperature conditions for sustained periods. This type of corrosion is of great concern in gas turbine engines, and even automotive combustion engines.

Dimensional Control
Applications for dimensional control, restoration and applications where a specific surface profile or contour is required. Coating can be used in their "as-sprayed" condition, or can be ground/machined to very tight dimensional tolerances and to a wide variety of surface finishes to suit application requirements.
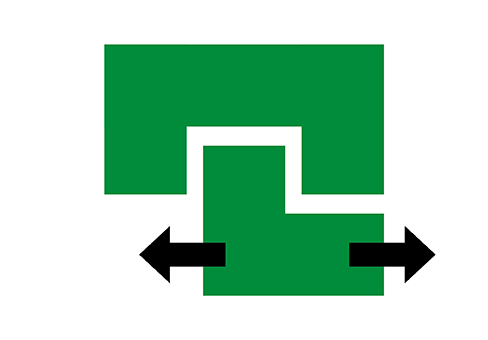
Clearance Control
In rotating equipment such as compressors, gas turbines and turbochargers, dimensional changes take place between the rotor and stator components because of thermal and mechanical effects during operation. These dimensional changes affect sealing by opening gaps between the blade tips and the casing in gas path systems, and between the seal and housing in labyrinth seal systems. In these applications, clearance control systems can be installed that consist of a sacrificial element and a cutting component. Special thermal spray coatings, called abradables, and honeycomb seals form effective sacrificial systems.

Salvage and Restoration
The use of the thermal sprayed coatings for the repair of mismachined or worn components is well known. The materials used for these coatings can either be metallurgically similar to the substrate material or of a material that will provide additional desirable surface characteristics, such as wear resistance. The coatings can be applied quite thick and are easily machined to dimension and the required surface finish.
Embossing and Engraving
- Industries that process plastic foils or paper frequently need to produce surfaces with an attractive irregular, matte appearance. To create the desired surface texture, the foil or paper is pressed between a textured embossing roll and a press roll. A thermal spray coating applied to a new or used embossing roll can produce a surface having a homogeneously, random pattern.
- Within the printing industry, thermal spray coatings are a mainstay for anilox rolls. A dense, homogeneous coating of chrome oxide is applied to the roll, which is subsequently laser engraved with a fine pattern. These coated rolls, used for ink transfer during the printing process, are both wear resistant and corrosion resistant to today’s water based ink formulas.
- The surface profile of corrugating rolls is maintained using wear resistant thermal spray or thin film coatings, with rolls of over four meters (13 feet) in length regularly coated.
Electrical properties
Electrical properties such as conduction, induction, insulation, magnetism, resistance heating can all be achieved using coatings. These coatings can be applied over very large areas, such as on corona rolls, or locally for sensor applications.
Conduction
Metallic coatings are applied to non-conductive or poorly conductive base materials to enhance electrical conductivity. It is also possible to apply combinations of conductive and non-conductive coatings. Such coatings are used to prevent static loading, to shield against electromagnetic radiation or simply to improve the conductivity of electrical contact areas.
Insulation
Ceramic materials are excellent electrical insulators when applied to metallic substrates. These coatings are stable at high temperatures, and resistant to molten metals and mechanical wear.
Magnetism
- Coatings of ferromagnetic materials (Fe, Ni, Co) are precisely applied to very small surfaces to produce an induction voltage that can be detected by a sensor to determine the exact position of a moving component. An example where magnetic coatings are used are for piston rods in contaminated environments.The surface profile of corrugating rolls is maintained using wear resistant thermal spray or thin film coatings, with rolls of over four meters (13 feet) in length regularly coated.

